The high strength alloys in the 2000 AlCu and 7000 AlZnMg are normally not recommended for use in seawater. Aluminum with pretty SS fittings is a battery generating 9V 247 in salt water.

During electrochemical corrosion electrons from other compounds are attracted to the metallic ions.
Aluminum and salt water corrosion. However salts are extremely corrosive. When salt air and salt water come into contact with aluminum they can cause both the chalky white coating of aluminum oxide and unpleasant pitting. Fortunately theres an easy way to protect aluminum from salt water and prevent unsightly corrosion.
Preventing Corrosion in Aluminum. AlMg and 6000 AlMgSi series are resistant to corrosion in sea water especially the so-called seawater resistant alloys in the 5000 series 1. The high strength alloys in the 2000 AlCu and 7000 AlZnMg are normally not recommended for use in seawater.
In support of the foregoing aluminum boats constructed from 5000-series alloys were already. The salt does not directly attack the aluminum but causes an electrochemical attack like a catalyst that results in the corrosion. This is the white deposit that you find on aluminum.
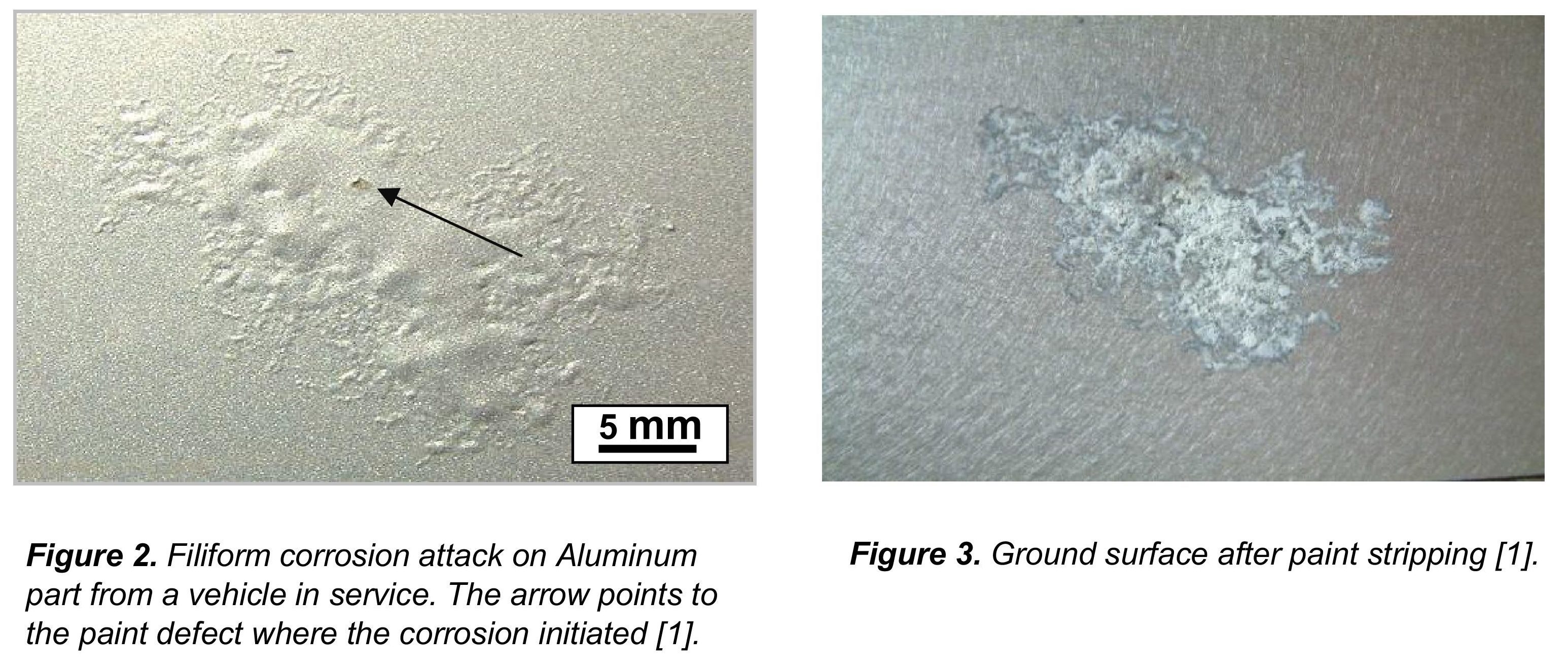
The reaction rate is usually fairly slow but on this 30 year old vessel the salt water had definitely taken its toll. Everbrite Protective Coating is a one part clear coating that will protect the aluminum and other surfaces from salt water corrosion. It will also protect from UV.
You can see more information by following the link to our website. Protecting Aluminum Boats From Salt Water Corrosion To prevent hull pitting caused by trapped moisture replace carpeted trailer bunks with plastic such as these plastic bunk covers from Load Rite Trailers. Slippery plastic also makes launching easier.
Salt water causes the corrosion of aluminum creating aluminum oxide. The salt does not directly attack the aluminum but causes an electrochemical attack like a catalyst that results in the corrosion. This is the white deposit that you find on aluminum.
The reaction rate is usually fairly slow but that depends on the alloy. When salt air and salt water come into contact with aluminum they can cause both the chalky white coating of aluminum oxide and unpleasant pitting. Fortunately theres an easy way to protect aluminum from salt water and prevent unsightly corrosion.
One form of corrosion that occurs when metal and saltwater get together is called electrochemical corrosion. Metal ions dissolve in water and saltwater conducts electricity and contains ions which attract ions from other compounds. During electrochemical corrosion electrons from other compounds are attracted to the metallic ions.
Its heavy which will limit your ability to fly and worse than that it rusts. Spend too long in damp environments and youll find reddish brown flakes lifting off the surface. Aluminum would be better because its much lighter and doesnt rust.
Aluminum does corrode though especially if. By pairing the natural corrosion resistance of aluminum with special coatings aluminum alloys prove to be valuable especially in salt water applications. When it comes to marine applications aluminum blows steel right out of the water.
From shipping vessels to personal watercraft aluminum alloys have staked their claim on the water. When aluminum is wetted with the saltwater and water enters the crevice little happens initially. Over time inside the crevice oxygen is consumed due to the dissolution and precipitation of aluminum.
Crevice corrosion can occur in a saltwater environment if the crevice becomes deaerated and the oxygen reduction reaction occurs outside of the crevice mouth. Under these conditions the crevice becomes more acidic and corrosion. The Chemical Nature of Aluminum and Aluminum Corrosion Aluminum is an amphoteric metal and can react with both chemically acidic and basic substances.
When freshly produced aluminum is highly reactive and reacts spontaneously with water andor air instantly forming a thin layer of aluminum oxide alumina on its surface. Aluminum with pretty SS fittings is a battery generating 9V 247 in salt water. On commercial vessels you rarely see SS in Aluminum but on pleasure boats it is a attractive common but damaging error in design.
Anodized or not the battery effect is relentless. Look up galvanic corrosion for details on prevention. The rate of corrosion of aluminium in water depends on several parameters coupled to water.
PH temperature electric conductivity elements in the water and movements of the water. The type of corrosion will also differ for different conditions. An increase in temperature results in a higher rate of chemical reactions.
In water at a higher. Aluminum boats require special care to prevent salt water corrosion. Theyre light economical nearly maintenance-free easy to repair and almost impervious to damage.
The Coast Guard and Navy prefer them for small craft and many commercial boats are aluminum as are many recreational craft. If metal can corrode around salt water does it make sense to use aluminum boats in a salt water environment. Aluminum boats in general and Duroboats in particular offer great performance ease of maintenance and economy.
The need to operate in salt water should not deny you those benefits but you must be smart about it. Resistant to clean water damage but not floodwater damage. Bare carbon steel can be more than 100x prone to corrosion than aluminum and more than 8000x greater than 316 stainless steel – more on that later.
Salt can break down the protective oxide that forms on aluminum. On a boat without any corrosion protection aluminum being the most active metal will become the anode and the stainless steel a less active metal will be the cathode. Electrons will flow from the anode to the cathode resulting in a loss of anode material visible as corrosion on the aluminum.
Given enough time however aluminum products can develop large holes caused by corrosion. This is why its important to prevent aluminum from corrosion. One way to protect aluminum from corrosion is to store it in a climate-controlled environment.
Corrosion is the result of environmental factors such as moisture triggering a chemical. The most common metal fasteners used to combat salt water corrosion are titanium Grades 2 and Grade 5 and Monel alloy 400 and K500. While some of the nickel alloys like Hastelloy are resistant to sea water they are generally overkill.
Selecting the best material for a salt water environment also requires you consider several additional variables.
