Since an electrolytic cell for cleaning artifacts lacks the resistance of a battery and the battery chargers generally do not have the necessary internal resistance controls to compensate for it the chargers run well above their maximum safe operating amperage. This reduced iron will form a somewhat porous layer of new iron on the metal object being cleaned.
During electrolysis the rust turns from orange to black.
Electrolysis but battery charger has safety. You can hook the charger to a fully charged battery then use jumper cables to attach it to your Etank. The charger will keep your battery while it runs the etank for a few hours. The battery WILL be completely died by the end.
It might be long enough to make scrubing easier. Here is the rub. With a budget charger using a lot of electrolyte will drive your charger at the maximum capacity.
Lower cost chargers are not rated for 100 duty cycle and may burn it out with time so in this case too much conductivity is not a good thing. Electrolysis has been used to separate many elements from impurities present in their natural form since the 17th century. Charger cableswith safety clamps.
Ammeter if using a car battery and not a charger Wire brush. Unplug the batterycharger and use the ammeter to set it at 2 amps. Findoggy - a self exciting charger has output all the time.
The newer style or automatic chargers must sense a bit of battery voltage before they will start. That prevents trying to charge a totally dead or damaged battery. If you have an older charger it is most likely manual and has.
Any AC power that gets through the charger into your electrolysis tank slows down or can even stop the process. If your system does not seem to work well try putting a 12-volt car battery in the circuit. All automotive battery chargers convert 120 volts Alternating Current AC to about 14 volts Direct Current DC for charging.
Battery charger a suitable charger that is able to produce a minimum of 6 to 10 volts DC. Metal rods for smaller sized jobs but we suggest a large sheet of metal to speed up the process. Arm and Hammer laundry soda also referred to as washing soda.
Wire andor cables for connecting electrodes. During electrolysis the rust turns from orange to black. In most cases the rust next to the iron is reduced to iron metal.
This reduced iron will form a somewhat porous layer of new iron on the metal object being cleaned. After electrolysis the iron object will rust very. The battery charger has to have a manual switch.
I found this one at a thrift shop for 1999. I thought that was a good price so I scooped it up. If you cant find a manual charger you would need a 12v automobile battery and booster cables.
An automatic charger would power down if no battery was present. A manual one will pump out 12 volts. Electrolysis removes rust from metallic objects.
During electrolysis an electrical current will flow through a liquid such as a molten ionic compound or aqueous solution. Atoms are rearranged causing irreversible changes in chemical composition. Adding conductors often in the form of copper wire which has a higher melting point to the.
Since an electrolytic cell for cleaning artifacts lacks the resistance of a battery and the battery chargers generally do not have the necessary internal resistance controls to compensate for it the chargers run well above their maximum safe operating amperage. Safety Precautions How to Remove Rust from Tools Electrolysis Ensure that no spill can reach the battery charger potential for electrocution as with any electrical appliance. The charger cables are relatively safe but you may still receive a slight shock if you put your hands in the solution or touch the electrodes while the charger is operating.
This procedure is approved by the FDA and has been determined as a safe and permanent method to remove unwanted hair. Electrolysis is extremely versatile. It can be used on every part of the body including eyebrows face arms legs and the very sensitive bikini area with minimal adverse side effects.
The most common side effect is redness and. This is a method of rust removal that is effortless and effectiveWarning. Do not do this inside.
Hydrogen gas is produced and can ignite in enclosed spaces. Remove flames cigarettes etc. Use gloves and avoid touching the electrodes.
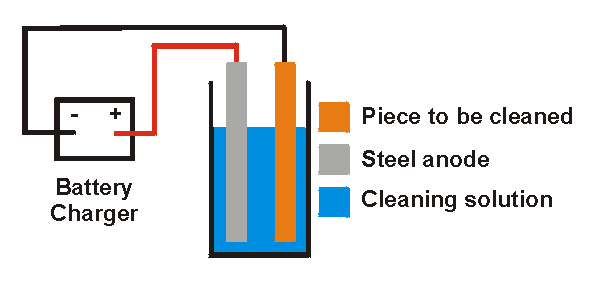
The electrolysis method involves putting water into a suitable heavy-duty plastic container and adding either washing soda or lye. One wire from a battery charger is hooked up to an anode placed in the water and the other wire from the battery charger is hooked up to the metal piece from which the rust is. Modifications made to my Harbor Freight battery charger for electrolysis and other projects.
If you want to support me and future projects do so athttps. Also the battery charger you use must be a manual one or have a manual charge mode. An automatic charger will see the electrolysis tank as a charged battery and shut itself down.
If you already own a fully automatic charger and dont wish to purchase a manual one there is a workaround although it necessitates the use of a 12V car battery. It is not recommended for brass aluminum copper orexotic metals and alloys. Electrolysis is a method of removing iron oxide by passing asmall electrical charge from a battery or battery charger through the rustymetal to stimulate an exchange of ions while the tool is submerged in anelectrolyte solution.
Electrolysis is a method used to remove rust from metal parts. All information illustrations and specificationsare based on the best information available at the time of publication. This article has been reproduced with the permission of the original author.
Please respect all copyright issues. Once everything was good to go I turned the timer on my charger has a mechanical charge timer and watched for the reaction. Almost immediately a storm of white bubbles began to surface from around the cultivator shank.
As mentioned earlier the battery charger ammeter read about 8 amps. I watched this for awhile and left it for about 15. The gasses released when a battery is bubbling due to overcharging electrolysis can be dangerous in a confined area without proper ventilation.
Hydrogen is released which can explode with flame or spark and hydrogen sulfide is noxious when inhaled. Basic SAFE Electrolysis Volume 1 - YouTube.
